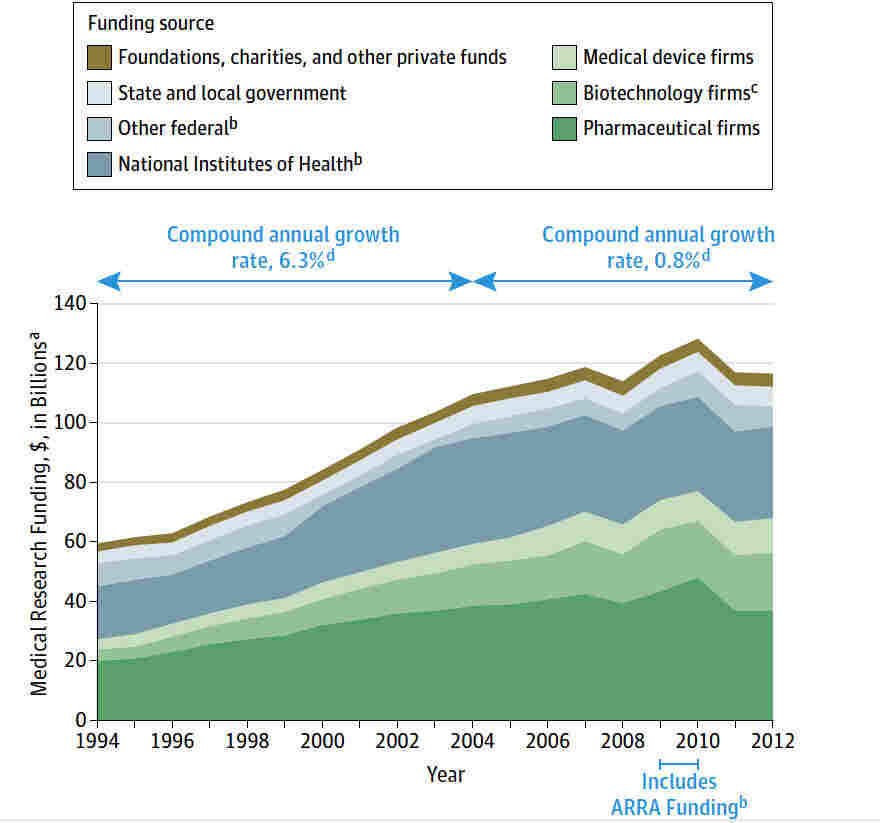
Medical research funding plays a pivotal role in safeguarding patient wellbeing during clinical studies. Recent federal funding cuts have revealed the critical nature of financial support in ensuring patient safety in research environments. These interruptions not only stall innovative projects but also hinder the effectiveness of Institutional Review Boards (IRBs) responsible for overseeing ethical compliance and protecting research participant rights. Without adequate resources, essential oversight mechanisms may falter, leading to potentially harmful consequences for individuals involved in studies. In this increasingly complex landscape of clinical trial ethics, it is imperative to acknowledge how NIH funding impacts the overall safety and efficacy of medical research.
Investments in medical research are crucial for maintaining high standards of ethical oversight within clinical studies. The allocation of resources to ensure proper governance, such as through funding aimed at the oversight of institutional review boards (IRBs), cannot be understated. When funding streams falter, it directly affects the scrutiny placed on research proposals, which ultimately safeguards the wellbeing of participants. Moreover, the ongoing support for ethical considerations in multisite studies further reinforces the rights of those involved in medical research, fostering trust and transparency. As the medical research landscape evolves, maintaining financial backing is essential for upholding the safety and rights of those who volunteer for potentially life-changing studies.
Impact of Funding Cuts on Patient Safety in Research
The recent funding cuts imposed by the Trump administration have severely disrupted ongoing research activities across institutions, including pivotal programs designed to ensure patient safety. With over $2 billion in federal research grants frozen, vital oversight mechanisms are at risk, severely compromising patient protection protocols. The halt in funding affects various critical areas, specifically those relating to the rights and well-being of patients participating in clinical trials. Organizations work tirelessly to mitigate potential risks in research, but without adequate funding, their ability to maintain patient safety standards diminishes significantly.
With the SMART Institutional Review Board (IRB) system encountering funding disruptions, the implications for patient safety are profound. These interruptions can lead to unchecked experiments, where the ethical considerations and safeguards that IRBs provide could fall by the wayside. The rigorous oversight provided by these boards is designed to protect human subjects from harm, ensuring that their rights are respected, and any adverse events are carefully monitored. As funding sources dwindle, the community’s reliance on volunteer participants in medical research could also wane, potentially leading to a public distrust in clinical studies that is detrimental to health advancements.
The Role of NIH Funding in Protecting Research Participants
NIH funding plays a crucial role in safeguarding research participants’ rights through comprehensive reviews and approvals mandated by Institutional Review Boards (IRBs). These boards are vital in maintaining ethical standards, ensuring that research complies with local, state, and federal regulations. The introduction of new policies requiring multisite studies to be reviewed by a single IRB streamlines this oversight, allowing for more efficient research while still prioritizing participant safety. However, as funding cuts threaten the operational capacity of research institutions, the ongoing integrity of these oversight processes hangs in the balance.
Furthermore, NIH funding impacts the training of researchers and the development of innovative practices that safeguard participants’ interests. With adequate funding, institutions can enhance their research ethics programs, fostering a culture of accountability that prioritizes patient safety and informed consent. As the landscape of medical research continues to evolve, it is essential that financial support from NIH is consistent to preserve and advance the ethical frameworks that protect research participants. Cuts to such funding not only undermine these essential programs but also risk the potential progress in scientific discovery.
The Significance of IRB Oversight in Clinical Trials
Institutional Review Boards (IRBs) serve as a regulatory backbone for clinical trials, evaluating proposals to ensure they meet ethical standards, prioritizing the safety and rights of participants. The IRB process consists of comprehensive assessments that include evaluating study designs, potential harm, and ensuring that informed consent is adequately obtained and maintained throughout the research duration. The presence of a robust IRB protects participants from ethical violations, ensuring their engagement in research is voluntary and informed.
As the landscape of clinical trials continues to expand and diversify, the role of IRBs becomes even more critical. They are tasked with adapting to new technologies and research methodologies while preserving participant safety. Moreover, the IRBs are not just gatekeepers of ethics; they also improve public trust in research by ensuring that trials are conducted with the utmost respect for participants’ rights and well-being. The interruption of funding threatens these essential functions, highlighting the need for secure funding structures to support research ethics and protect human subjects.
Understanding Research Participant Rights and Protections
The rights of research participants are paramount in clinical studies, safeguarded by federal regulations and ethical standards that set the groundwork for ethical clinical research. Participants have the right to be informed about study procedures, risk factors, and potential benefits. Informed consent is a cornerstone of ethical research practices that all studies must adhere to, ensuring participants have a clear understanding and voluntarily agree to participate. However, without the necessary funding, institutions may struggle to maintain these essential practices, putting participants’ rights at risk.
Moreover, a conscious effort must be made to ensure that diverse populations are represented in clinical trials. The commitment to protecting research participants extends to understanding the unique needs of various demographic groups and how their rights may differ under study conditions. Funding cuts can impede the capacity to advocate for vulnerable populations, thereby disrupting efforts to build equitable research environments. As institutions navigate these challenges, reinforcing the importance of research participant protections is more crucial than ever.
Ethical Challenges in Clinical Trials and Funding Cuts
Ethical considerations in clinical trials have been shaped by historical abuses and the need for rigorous oversight. Funding cuts threaten to reverse the progress made in embedding ethical oversight, as IRBs and their regulatory frameworks depend on adequate resources to function optimally. These boards must remain vigilant and adaptive to the changing research landscape, and without sufficient financial backing, the ethical rigor that protects participants may wane. A failure to uphold these standards may result in a repeat of historical missteps, potentially eroding public confidence in research.
Additionally, the ethical challenges surrounding patient safety, informed consent, and risk assessment are compounded by restrictive funding environments. Researchers often face pressures to produce results quickly amid financial constraints, which can lead to ethical lapses. The potential for compromised participant welfare in pursuit of expedited outcomes raises significant concerns within the research community. Ensuring strong ethical oversight through well-supported IRBs is essential to safeguard the rights and health of participants, reinforcing the critical need for stable research funding.
Addressing Public Trust in Medical Research Amid Funding Challenges
Public trust in medical research is fragile and must be carefully cultivated, especially in light of disruptions caused by funding cuts. Transparency, ethics, and accountability are paramount to maintaining trust, but these can only be upheld through adequate resources and support systems. The ongoing freeze of critical research funding can inadvertently foster public skepticism towards research initiatives and institutions, hindering recruitment for important studies. Trust among study participants is crucial for the successful advancement of research, as it relies heavily on participants feeling valued and protected.
Moreover, engagement strategies that promote transparency and demonstrate commitment to participant welfare are vital in restoring public confidence. Researchers must communicate openly about how funding affects their capacity to ensure patient safety and uphold ethical standards. Initiatives that emphasize patient rights and protections should be prioritized to encourage a culture of trust, demonstrating to the public that their safety and well-being are the forefront of all research endeavors. Addressing these challenges requires a collaborative effort among researchers, institutions, and policymakers to prioritize funding that sustains ethical public health endeavors.
Long-term Consequences of Research Funding Cuts
The long-term ramifications of cutting research funding could be devastating, not only for patient safety but also for the landscape of medical innovation. As studies are discontinued or delayed due to financial constraints, critical advancements in treatment and healthcare could stall. These cuts represent not just a temporary setback but a potential period of prolonged stagnation in vital clinical research that could benefit countless individuals suffering from various health conditions.
Furthermore, diminished funding threatens the sustainability of research programs that focus on ethical oversight and participant protections. Ensuring that research is conducted responsibly is not only foundational to public health but also essential in fostering a robust research culture. Long-term impacts may include a decrease in participant enrollment due to diminished public confidence, resulting in fewer advancements in public health interventions and medications. Committing to secure research funding is essential for reversing this trajectory and prioritizing the health and safety of all citizens.
Reinforcing Patient Safety Standards through Collaborative Research
Collaborative research initiatives are crucial for advancing clinical studies while upholding patient safety standards. By pooling resources and expertise across institutions, researchers can leverage shared insights to enhance patient welfare in trial settings. The SMART IRB system exemplifies the power of collaboration, allowing multiple institutions to participate in studies while ensuring that all patient safety protocols are uniformly enforced. However, with funding cuts jeopardizing collaboration, the capacity for seamless integration across sites may falter, ultimately impacting patient care.
Additionally, fostering a collaborative environment requires commitment to funding mechanisms that support joint ventures among institutions. This not only strengthens research oversight but also enriches the ethical landscape of clinical investigations. As the impetus for collaboration grows amid budgetary constraints, prioritizing patient safety and ethical compliance is essential. Addressing these challenges through innovative funding models and partnerships is vital for sustaining robust, safe research practices that prioritize the well-being of all participants.
Future Directions in Ensuring Patient Rights and Safety in Research
As the medical research landscape evolves, the need for comprehensive frameworks to ensure patient safety and rights becomes increasingly essential. Future initiatives must focus on advocating for sustainable funding sources that enable continuous support for ethical oversight in clinical trials. Balancing innovation with patient protection requires a concerted effort from funding bodies, research institutions, and oversight committees to reinforce confidence in clinical studies. Additionally, integrating patient feedback into the research process will enhance ethical standards and ensure that participant needs are adequately met throughout study participations.
Furthermore, advancements in technology can play a pivotal role in safeguarding patient rights. The incorporation of digital tools for informed consent and ongoing communication can improve transparency and participant engagement. Anticipating future challenges in patient safety and participant rights should be at the forefront of research agendas, as proactive strategies can mitigate potential issues before they arise. Ensuring a future of robust, ethical medical research depends on collaboration, adequate funding, and a steadfast commitment to protecting the individuals who contribute to progress.
Frequently Asked Questions
How does medical research funding impact patient safety in research?
Medical research funding directly supports essential oversight mechanisms like Institutional Review Boards (IRBs), which ensure patient safety in research. Funding provides the necessary resources for IRBs to review and approve research proposals, monitor clinical trials, and uphold research participant rights. Without adequate funding, these protective measures could diminish, leading to increased risks for patients enrolled in clinical studies.
What role does NIH funding play in enhancing participant rights in medical research?
NIH funding is crucial for maintaining the ethical standards of clinical trials, which includes safeguarding the rights of research participants. Through grants, the NIH provides support for the necessary infrastructure that facilitates IRB oversight, ensuring that studies comply with regulations designed to protect patient welfare and informed consent processes.
How does IRB oversight relate to medical research funding and patient safety?
IRB oversight is a critical component of the medical research funding process. Medical research funding enables IRBs to operate effectively by providing resources to review research proposals thoroughly, ensure adherence to ethical standards, and protect patient safety throughout clinical trials. Cuts in funding can jeopardize IRB functions, potentially compromising participant safety.
What are the ethical considerations in clinical research funding related to patient involvement?
Ethical considerations in clinical research funding focus on ensuring that participant rights are upheld, informed consent is obtained, and the risks of harm are mitigated. When medical research funding is robust, it supports thorough ethical reviews by IRBs, which are vital for protecting the safety and autonomy of research participants, thus fostering trust in the research process.
What are the potential consequences of funding cuts on patient safety in clinical trials?
Funding cuts can severely impact patient safety in clinical trials by limiting IRB oversight and resources needed for effective monitoring of research. This could lead to inadequate review processes, increased risks during trials, and ultimately, a potential loss of public trust in medical research. Ensuring that funding remains stable is essential for maintaining patient safety and ethical standards.
| Key Point | Details |
|---|---|
| Impact of Funding Cuts | Federal funding cuts disrupt important oversight of medical research, risking patient safety. |
| Role of IRBs | Institutional Review Boards (IRBs) ensure the rights and welfare of research participants are protected. |
| Significance of Collaboration | Collaboration through systems like SMART IRB simplifies the research process across institutions. |
| Historical Context | Past ethical violations in research underline the need for stringent oversight and patient protections. |
| Consequences of Halting Studies | Halting studies can lead to mistrust in research, risking participant health and community relations. |
Summary
Medical research funding is crucial for the safety and well-being of patients involved in clinical studies. The halt in these funds not only jeopardizes the integrity of ongoing research but also endangers the very individuals it aims to benefit. As demonstrated, the suspension of over $2 billion in federal research grants has led to considerable disruption in oversight mechanisms, particularly through the work of Institutional Review Boards (IRBs) that safeguard participant rights. Without adequate funding, many studies face delays, and the risk of ethical violations rises, undermining public trust in medical research. It is essential to restore and prioritize medical research funding to sustain effective oversight and protect patients.