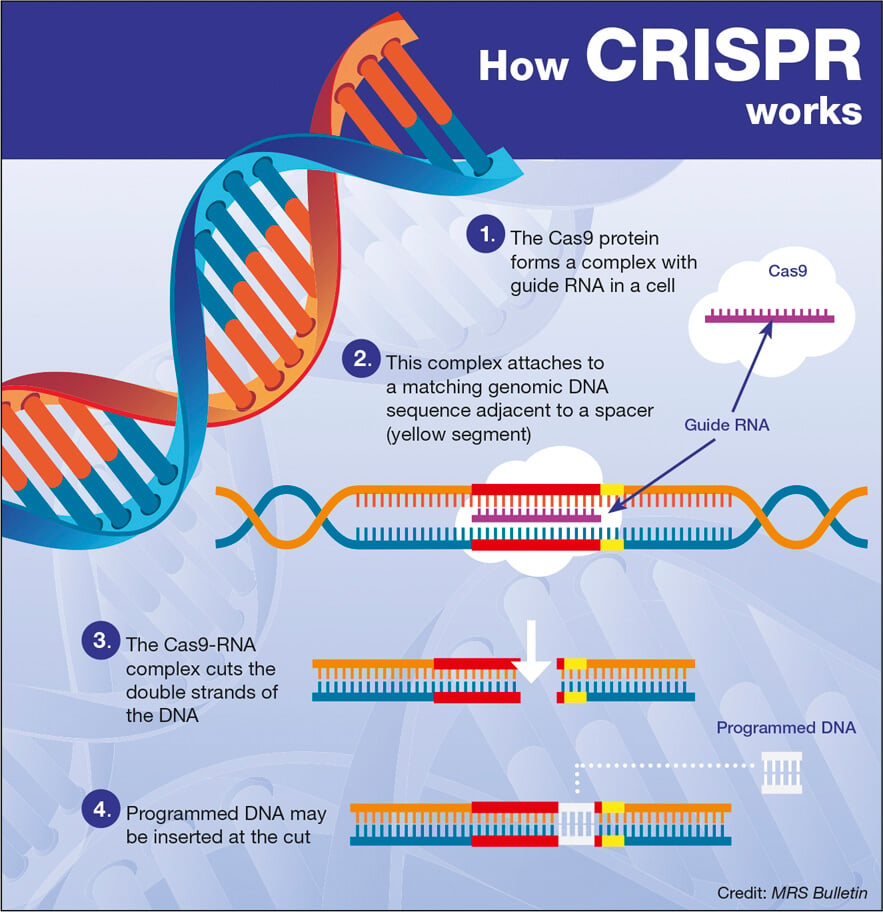
CRISPR gene editing is revolutionizing the field of biotechnology by enabling precise alterations to DNA, which could potentially lead to cures for genetic disorders like sickle cell disease. This groundbreaking technology empowers scientists to manipulate genes with unprecedented accuracy, raising significant conversations about the ethics of gene editing. As researchers explore the possibilities of CRISPR technology, important questions about bioethics and health equity in gene therapy come to the forefront. While the prospect of using gene editing to eliminate devastating diseases ignites hope, it simultaneously prompts scrutiny over gene editing ethics and the equity of access to such therapies. The dual edges of innovation and ethical responsibility present a complex landscape for the future of medicine and genetics.
Gene editing methodologies, particularly those represented by CRISPR technology, are making waves in medical research and treatment. This genetic manipulation tool allows for intricate edits within the genome, promising advancements in therapies for conditions like sickle cell anemia. However, the discussions surrounding the morals and implications of such technology highlight the pressing need for a deeper understanding of bioethics related to genetic modifications. As we navigate the potentials of this powerful tool, we must address key concerns regarding fairness and health equity in access to these innovations. Ultimately, reconciling the promise of these advancements with the ethical dilemmas they pose will be critical in shaping the future of healthcare.
The Ethical Landscape of Gene Editing
The field of gene editing, particularly through CRISPR technology, is rife with ethical quandaries. As we stand on the cusp of remarkable advancements that could potentially eradicate genetic diseases, the philosophical debate surrounding these innovations intensifies. Questions arise about the implications of manipulating human genetics — whether we possess the moral authority to adjust characteristics that could define an individual’s identity. Renowned figures in bioethics emphasize the necessity of thorough discourse when confronted with such cutting-edge technology, underscoring the potential risks associated with altering human genes.
Baer and Brendel highlight the intricate balance needed between scientific progress and ethical responsibility. The nuances of gene editing ethics demand careful examination, especially as we ponder the broader societal impacts. For instance, if gene editing can eliminate conditions like sickle cell disease, should this technology also be used for enhancing so-called non-pathological traits? These profound dilemmas note a crucial intersection of medicine and morality, and it’s essential to engage various stakeholders—including ethicists, scientists, and the public—in this ongoing conversation.
CRISPR Technology and Sickle Cell Treatment
CRISPR gene editing has emerged as a beacon of hope for many genetic disorders, notably sickle cell disease, which affects thousands in the U.S. and millions around the globe. The cutting-edge simplicity of this technology allows doctors to pinpoint and correct flawed genes within a patient’s DNA, potentially curing debilitating conditions that would otherwise result in chronic suffering and early mortality. This therapeutic approach offers unprecedented opportunities for advancing human health, and the prospect of alleviating sickle cell symptoms and restoring the quality of life is particularly transformative.
However, the high costs associated with CRISPR treatments present significant hurdles, raising critical questions about healthcare equity. As Baer pointed out, the staggering price tag of around $2.2 million for sickle cell treatment will likely limit access to those who can afford it, thereby exacerbating existing disparities in health equity. Ensuring that such groundbreaking therapies are equitably available is paramount, as they should benefit not just a privileged few but equally address the needs of all patients afflicted by genetic conditions.
The Debate on Genetic Modification and Health Equity
The conversation surrounding CRISPR gene editing extends beyond medical capabilities; it fundamentally ties into the principles of health equity and justice. As innovative treatments emerge, the disparities in access and affordability cannot be overlooked. Bioethicists, like Brendel and Baer, stress the importance of including broader community perspectives in discussions surrounding gene therapies. They warn that without careful thought, new scientific advancements may only deepen the chasm between different socioeconomic groups, potentially making healthcare a privilege for the wealthy rather than a right for all.
This prospect of deepening inequality raises pressing moral questions about who benefits from these technologies and who might be left behind. As capabilities like genetic editing grow, it becomes crucial to champion policies and frameworks that promote access and equity. Public health initiatives must ensure that every individual—regardless of their background—is afforded the potential benefits of gene editing enhancements, reinforcing a healthcare system built on fairness and justice.
Understanding the Bioethics of CRISPR and Human Variation
The bioethics of CRISPR technology intersects with broader societal views on human variation and identity. As touched upon in discussions about editing out genes linked to genetic disorders, such as deafness or albinism, significant ethical considerations arise regarding the definition of health and the Acceptance of diversity in human ability. Statements from advocates in the deaf community challenge the dominant narratives surrounding physical traits, emphasizing that variation does not equate to pathology, and that diversity should be celebrated rather than eradicated.
Exploring the bioethics of gene editing invites a deeper understanding of what it means to be human in a rapidly advancing technological landscape. As we harness the power of CRISPR to edit genes, there must be an ongoing dialogue regarding what ‘modifications’ we deem acceptable and who decides these parameters. Balancing the desire for technological progress with the respect for human differences remains a complex yet essential part of the conversation in gene editing.
Balancing Innovation and Oversight in Gene Editing
As gene editing technologies develop more rapidly than regulatory frameworks can adapt, the issues of oversight and accountability become increasingly pressing. Baer emphasizes that while certain practices, like germline editing, are legally restricted, the reality of enforcement raises significant questions about international compliance and ethical governance. Countries with fewer regulatory restrictions might pave the way for unchecked experimentation, potentially leading to unforeseen consequences.
The necessity for robust international guidelines is crucial to ensure that advancements in CRISPR and gene editing technologies do not compromise human dignity or safety. Innovative solutions must also include public discourse and comprehensive ethical assessments to guard against possible abuses or unintended repercussions. The intersection of innovation and oversight demands a careful strategy, ensuring that while we embrace scientific advancements, we maintain strict ethical standards to protect future generations.
Potential Risks of CRISPR and Gene Editing
While CRISPR technology presents remarkable advantages for treating genetic disorders, the potential risks connected with its application require vigilant consideration. Baer highlights the intricate web of genetic interactions that may lead to unforeseen complications, suggesting that modifying a single gene could inadvertently affect numerous other biological functions. The ecological implications of these interventions remind us that meddling with the fundamental building blocks of life comes with moral and practical responsibilities.
Addressing these risks means emphasizing the importance of comprehensive testing and ethical assessments before implementing gene-editing solutions on of a wider scale. Scientific innovation must be paired with caution to navigate the unpredictable nature of genetic manipulation and to safeguard the surrounding ecosystem. The exploration of gene editing thus necessitates interdisciplinary approaches, engaging scientists, ethicists, and policymakers in discussions about potential hazards.
Public Perception of Gene Therapy and CRISPR
Public perceptions surrounding gene therapy and CRISPR can significantly influence the direction of research and implementation. As advances are made, widespread fear or misunderstanding can hinder acceptance of genetic editing techniques. Education and transparency are critical in demystifying gene editing, helping the public to recognize its potential benefits and ethical implications. Through effective communication, stakeholders can foster a balanced view that appreciates the promise of CRISPR alongside its risks.
Enhancing public knowledge can pave the way for more informed debates about the ethical aspects of gene editing technologies. When communities are equipped to engage with the complexities and consequences of genetic manipulation, they are better positioned to advocate for equitable access to treatments and safeguards against misuse. Establishing informed public discourse surrounding CRISPR gene editing fosters a more inclusive dialogue about its future applications.
Exploring Alternatives to CRISPR in Genetic Research
In the realm of genetic research, while CRISPR gene editing has gained considerable attention, alternative approaches are also being explored. Techniques such as TALENs (Transcription Activator-Like Effector Nucleases) and ZFNs (Zinc Finger Nucleases) offer distinct methodologies for targeting genetic sequences with precision. These alternatives may complement or serve as backups to the CRISPR technique, providing researchers with varied tools to approach genetic modification without solely relying on one specific method.
Research into these alternative tools remains essential so that the scientific community can harness an arsenal of techniques adaptable to diverse genetic challenges. Each method bears its own advantages and risks, and their exploration expands our understanding of genetic engineering. Ultimately, diversifying the tools at researchers’ disposal ensures a more balanced strategy in approaching gene therapy, fostering innovation while keeping ethical implications at the forefront.
The Future of Gene Editing and Human Health
As the possibilities of CRISPR gene editing unfold, the future of human health is being redefined. The intersection of technology and medicine holds the potential not only to eradicate genetic disorders but to enhance overall well-being. Looking ahead, the ability to tailor treatments based on individual genetic make-up opens doors for personalized medicine, which can revolutionize how we address various health conditions.
However, realizing this future hinges on responsible science and ethical guidelines that prioritize health equity. The groundwork must be laid now to ensure that the benefits of gene editing reach across socioeconomic barriers, offering hope to all patients, particularly those marginalized by health disparities. As we navigate the exhilarating and complex landscape of gene editing, ongoing discourse will shape the trajectory of this promising technology, ensuring it serves society as a whole.
Frequently Asked Questions
What are the ethical implications of CRISPR gene editing?
The ethical implications of CRISPR gene editing are significant and multifaceted. They encompass issues like the potential for creating ‘designer babies,’ where parents could select genetic traits for their offspring, raising questions about parental rights and societal norms. Additionally, CRISPR’s ability to edit germline DNA poses concerns regarding unforeseen long-term consequences on human genetics and biodiversity. Researchers emphasize the need for robust bioethics frameworks to guide CRISPR applications, ensuring that innovation does not outpace ethical considerations.
How is CRISPR technology used for treating diseases like sickle cell anemia?
CRISPR technology offers a groundbreaking approach to treating diseases like sickle cell anemia by allowing scientists to edit specific genes responsible for the disease. This gene editing technique can target somatic cells to remove and replace faulty genes, effectively curing individuals. Recent advancements have demonstrated CRISPR’s potential to not only treat but potentially eradicate sickle cell disease at a genetic level, raising both hope and complex ethical questions about access and implementation.
What is the role of health equity in gene therapy using CRISPR?
Health equity plays a critical role in gene therapy using CRISPR technology, as these innovative treatments often come with high costs, potentially limiting access for underprivileged populations. The disparity in availability of CRISPR-based therapies raises ethical concerns about who benefits from genetic advancements, emphasizing the need to ensure that all individuals, regardless of socioeconomic status, can access such life-saving treatments. Advocates for health equity stress the importance of developing policies that promote fair distribution of gene therapy.
Who should make decisions regarding gene editing for disabilities or genetic variations?
Decisions regarding gene editing for disabilities or genetic variations, such as those related to Down syndrome or deafness, present challenging ethical dilemmas. These questions often revolve around parental rights versus societal implications of ‘normalcy.’ It is crucial to involve a diverse array of stakeholders, including ethicists, medical professionals, and representatives from affected communities, to ensure that decisions reflect a broader understanding of human diversity and to avoid stigmatizing individuals with disabilities.
What are the concerns about oversight in CRISPR gene editing on a global scale?
Concerns about oversight in CRISPR gene editing on a global scale revolve around the regulation of genetic modifications, particularly in countries where enforcement may be lax. The potential for countries to experiment with genetic alterations without proper ethical oversight raises fears about ‘rogue’ applications of CRISPR technology, including its use in military or enhancement contexts. This underscores the need for international collaboration and governance to establish comprehensive guidelines that safeguard against unethical use of gene editing.
Can gene editing with CRISPR lead to unintended consequences?
Yes, gene editing with CRISPR can lead to unintended consequences, as genes can have complex interactions within biological systems. While targeting a specific gene may seem straightforward, changes can impact multiple pathways and processes within the body, leading to unforeseen health issues. Experts emphasize the importance of thorough research and cautious application to understand potential side effects, as narrowing the focus to a single gene does not account for the intricate nature of genetic functions.
| Key Points |
|---|
| CRISPR gene editing can cure diseases like sickle cell anemia, raising ethical questions about its use. |
| It allows editing of somatic and germline genes, potentially eradicating inherited diseases. |
| Ethical dilemmas arise over editing traits in living individuals, like Down syndrome or traits such as hearing. |
| High costs (e.g., $2.2 million for sickle cell treatment) raise issues of health equity and accessibility. |
| Innovation in gene editing can exacerbate existing inequalities in health care. |
| The lack of global oversight poses risks of unethical practices in gene editing. |
| Unintended consequences of gene editing must be considered due to complex gene interactions. |
Summary
CRISPR gene editing is a revolutionary technology with the potential to cure genetic diseases like sickle cell anemia, but it raises critical ethical questions. The ability to alter genes in somatic and germline cells introduces complexities regarding what it means to be human, the implications for health equity, and the risk of unforeseen consequences. As we advance in this field, it is essential to navigate the ethical landscape carefully, ensuring that innovations benefit all of society rather than a privileged few.