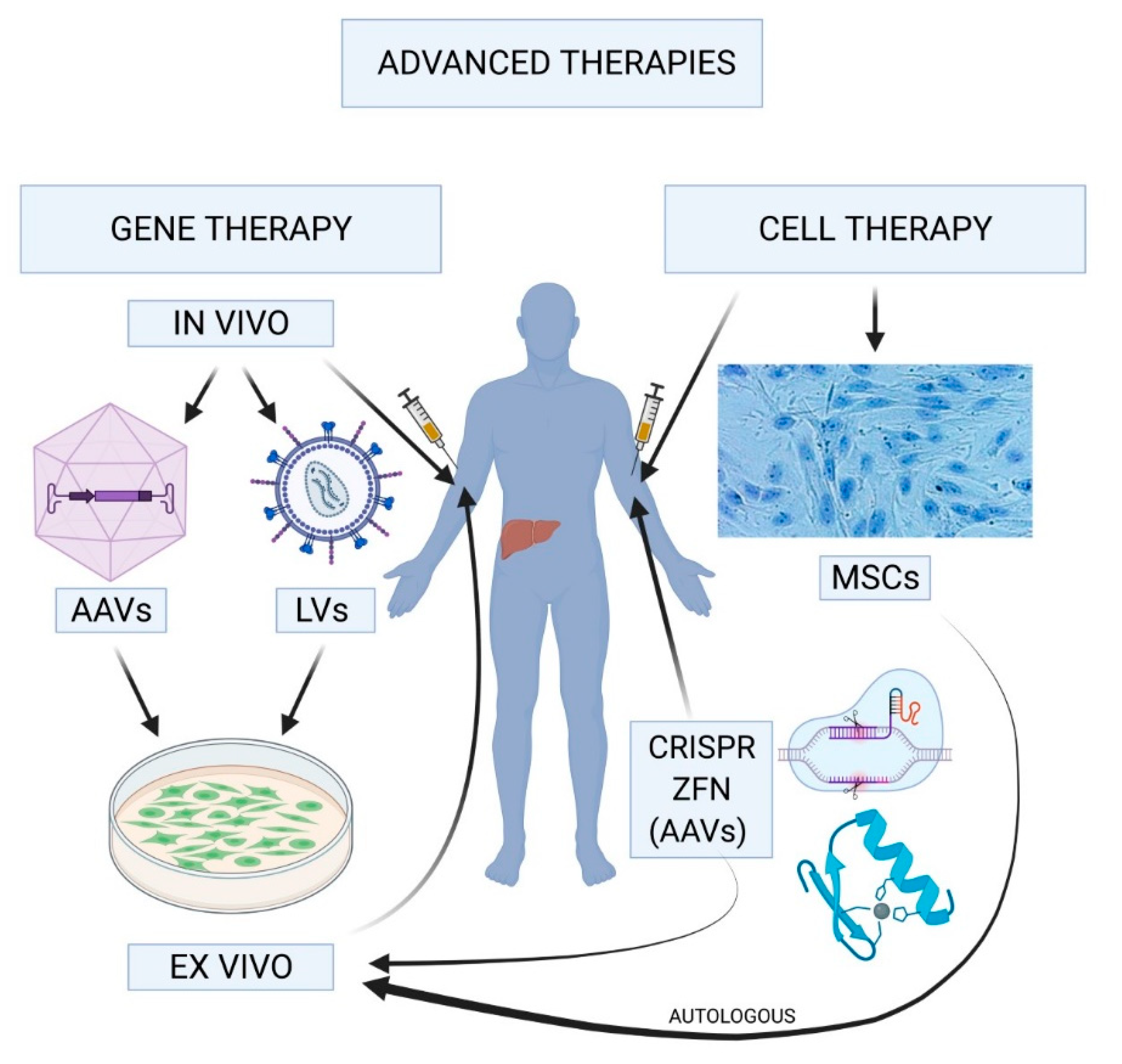
Gene therapy for hemophilia represents a revolutionary leap in hemophilia treatment, offering hope to those burdened by this bleeding disorder. This groundbreaking approach aims to correct the genetic defect responsible for the condition by delivering a functional clotting factor gene, specifically for individuals with hemophilia B. With the FDA-approved gene therapy known as Hemgenix, patients can potentially enjoy significant benefits, such as reduced reliance on regular injections of clotting factors. The advancements in gene therapy highlight not only the innovative techniques used to treat hemophilia but also the profound impact these innovations can have on quality of life. As more patients like Terence Blue experience transformational results, the field of gene therapy is set to reshape the narrative around hemophilia care and treatment.
The emergence of revolutionary treatments in genetic medicine offers a beacon of hope for individuals suffering from bleeding disorders like hemophilia. Also known as coagulation factor deficiency, hemophilia can significantly disrupt daily life, necessitating regular administration of clotting factors. Recent FDA-approved therapies, such as Hemgenix, exemplify the remarkable advancements in gene modification strategies aimed at restoring normal blood clotting function. The benefits of gene therapy extend beyond mere symptom management; they encompass a future where patients can lead more normal lives with minimal disruptions. As scientific exploration continues to unravel the complexities of genetic disorders, the potential for innovative treatments grows ever more promising.
Introduction to Hemophilia and Its Treatments
Hemophilia is a genetic disorder that impairs the body’s ability to make blood clots, which is essential for stopping bleeding. This condition predominantly affects males and results from mutations on the X chromosome, leading to a deficiency in clotting factors such as factor VIII or factor IX. Traditional treatment for hemophilia has involved regular injections of clotting factors, but advancements in medical science are giving hope to patients for more effective solutions. Among the latest breakthroughs is gene therapy, which aims to treat the underlying cause of hemophilia rather than just the symptoms.
Patients like Terence Blue have been managing hemophilia through these traditional methods for years, undergoing frequent infusions of clotting factors. However, with the advent of innovative treatments like Hemgenix, the landscape of hemophilia treatment is changing. Hemgenix, recently approved by the FDA, represents a new class of therapies designed to provide long-term solutions and has sparked discussions about the future of hemophilia care.
The Impact of Gene Therapy for Hemophilia
Gene therapy for hemophilia, particularly through products like Hemgenix, has garnered significant attention for its ability to potentially cure the condition by addressing the genetic deficiencies at their source. By delivering a functional copy of the gene responsible for producing clotting factor IX directly to the patient’s liver, this therapy aims to restore the body’s natural ability to create clotting factors that are essential for blood coagulation. This shift from regular injections to a one-time treatment could transform the lives of many hemophilia patients, allowing them to reduce their reliance on ongoing infusions of clotting factors.
The concept of gene therapy can be daunting for many patients, but the reassuring effectiveness demonstrated in nearly 94% of clinical trial participants who no longer required factor IX prophylaxis three years post-treatment illustrates this therapy’s potential impact. Terence Blue’s experience reflects this change, highlighting a future where patients could potentially live without the daily worry of managing severe bleeds. The benefits that gene therapy offers not only improve patients’ health prospects but also enhance their quality of life by minimizing the emotional and social burdens associated with managing hemophilia.
Understanding the Benefits of Hemgenix
Hemgenix offers remarkable benefits by potentially eliminating the need for patients to endure constant needle-based treatments. As a groundbreaking gene therapy specifically for hemophilia B, Hemgenix is revolutionizing hemophilia treatment paradigms, providing hope for a life less encumbered by frequent medical interventions. Patients treated with Hemgenix can expect to achieve significant and sustained increases in their clotting factor levels, translating to reduced bleeding events and minimized risk of joint damage over time.
Moreover, the FDA approval of Hemgenix signifies a broader acceptance and recognition of gene therapies in the treatment of genetic disorders. By showcasing its efficacy and safety, this therapy could inspire further advancements in the field, leading to new options for hemophilia A and other genetic conditions. The approval also reflects the evolving landscape of hemophilia treatment, where gene therapies stand to not only provide immediate health benefits but also valuable long-term economic advantages by reducing the overall costs associated with chronic treatment.
Challenges in the Adoption of Gene Therapies
Despite its potential, the market for gene therapies like Hemgenix faces significant challenges. The cost of such treatments remains a pressing issue, with Hemgenix listed at $3.5 million, despite insurance coverage typically reducing this price. The financial burden can deter healthcare providers and patients from embracing new options, particularly in a system that often prioritizes lengthy, chronic treatments over one-time genetic interventions. The hesitation to adopt gene therapies is compounded by a lack of understanding of their long-term benefits among patients and physicians alike.
Moreover, the relatively early stage of gene therapy development means that many patients are still acclimatizing to these advanced treatment options. Instances wherein therapies such as Pfizer’s Beqvez were withdrawn from the market due to low patient engagement underscore the importance of clear educational outreach and support. Advocating for gene therapies through education and patient testimonials could help mitigate skepticism and encourage more patients to consider these innovative solutions as viable alternatives to traditional treatments.
FDA Approval: A New Era for Gene Therapy
The recent FDA approval of Hemgenix signals a paradigm shift in hemophilia treatment, marking a crucial milestone in the application of gene therapy for genetic disorders. This approval paves the way for the introduction of additional gene therapies, enhancing patient access to groundbreaking treatments. It also establishes a framework for other drug makers to follow, encouraging more research and development in the field of gene therapy, particularly for rare conditions like hemophilia B.
Furthermore, the approval not only helps legitimize gene therapy approaches as feasible treatment pathways but also assists in shaping public perception. As more patients become aware of innovative treatments like Hemgenix, it is likely to foster a more favorable environment toward seeking advanced medical interventions. The potential for gene therapy to significantly shift the management of hemophilia is exciting, and continued research will help to bridge the gap between scientific potential and real-world application.
Managing Hemophilia Beyond Traditional Methods
While traditional hemophilia treatments have evolved significantly over the years, the need for a more radical approach is clear. For those living with this disorder, conventional treatments can often feel burdensome and disruptive to daily life. Innovations such as Hemgenix promise not just a reduction in treatment frequency, but an actual alteration in the clinical trajectory of hemophilia for many patients. Understanding that hemophilia management can extend beyond constant infusions opens new avenues for patient care.
Health practitioners and patients alike must remain educated and engaged in discussions around emerging therapies. As gene therapy continues to evolve, facilitating patient access and creating supportive environments for transitioning from traditional to novel therapies will be critical. The focus should be not only on providing immediate relief from bleeding episodes but also on enhancing the overall patient experience and quality of life.
The Future of Hemophilia Treatments
The future of hemophilia treatments appears promising, particularly with the advent of gene therapy. As researchers continue to unlock the mysteries of genetic modification and its applications in treating genetic disorders like hemophilia, the potential for more effective therapies will likely grow. Initiatives aimed at improving gene delivery mechanisms and creating safer, more effective therapy options are actively underway, suggesting a progressive trajectory for hemophilia management.
Moreover, broader discussions surrounding funding and support for gene therapy will be vital. Advocating for increased public and private investment in research dedicated to rare diseases like hemophilia can enhance the speed of technological advancements and approval processes within regulatory frameworks. Ultimately, the burgeoning field of gene therapy holds significant promise for radically reshaping how we understand and treat hemophilia and may ultimately lead to lives less burdened by the condition.
Building Awareness and Support for Gene Therapy
An integral part of promoting gene therapies like Hemgenix lies in increasing awareness among patients and healthcare providers. Educational campaigns can empower patients, enabling them to understand the profound implications that gene therapy can have on their lives. Educational initiatives should focus on demystifying gene therapy, providing straightforward explanations of how these treatments work, and emphasizing their long-term benefits.
Additionally, fostering support networks can play a significant role in patient engagement with innovative treatments. Patient testimonials, support groups, and collaborations between medical professionals can help create a conducive atmosphere for discussing gene therapy options. By building a supportive community that encourages open dialogue around these advancements, more patients may feel inclined to explore new treatment avenues and break the cycle of conventional treatment limitations.
Conclusion: A New Hope for Hemophilia Patients
The emergence of gene therapy as a treatment for hemophilia B signifies a turning point in the management of this genetic disorder. By providing patients like Terence Blue with options that can potentially reduce the burden of lifelong treatments, gene therapies present a new frontier in medical care. The hopeful outcomes observed in patients treated with Hemgenix underscore the transformative potential of these therapies.
As the medical community continues to explore and expand the use of gene therapy for hemophilia and beyond, the overarching goal remains clear: to enhance the quality of life for those living with genetic disorders. The journey from consistent, invasive treatments to a more stable, effective solution holds immense promise, paving the way for a future where hemophilia can be managed with unprecedented efficiency and success.
Frequently Asked Questions
What is gene therapy for hemophilia and how does it work?
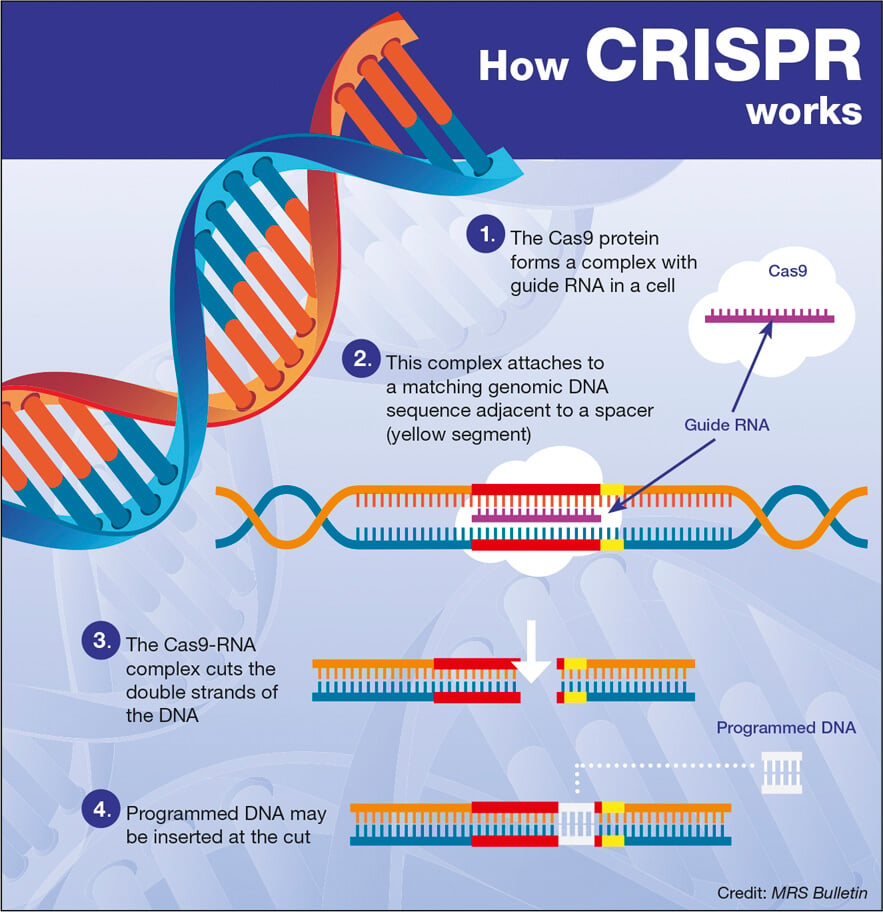
Gene therapy for hemophilia involves the use of gene editing techniques to correct the hereditary defects that cause the disease. Specifically, it aims to introduce a corrected copy of the gene responsible for producing clotting factor IX into the patient’s liver cells. This process enables the body to produce its own clotting factor, potentially reducing the symptoms of hemophilia significantly.
What are the benefits of gene therapy for hemophilia treatments like Hemgenix?
Gene therapy treatments such as Hemgenix offer several benefits, including the potential for long-lasting effects, reduced dependence on regular clotting factor infusions, and improved quality of life. Studies have shown that many patients treated with Hemgenix no longer require prophylactic treatment after a reasonable timeframe, marking a significant advancement in hemophilia management.
Is Hemgenix FDA approved and what does that mean for hemophilia treatment?
Yes, Hemgenix is FDA approved as a gene therapy for hemophilia B. This approval means that because it has undergone rigorous clinical testing and evaluation by the FDA, it is considered safe and effective for patients seeking to manage their hemophilia. The approval also facilitates insurance coverage for the treatment, making it more accessible to a larger patient population.
How does gene therapy for hemophilia compare to traditional hemophilia treatments?
Gene therapy for hemophilia offers a more permanent solution compared to traditional treatments that involve regular injections of clotting factors. While traditional treatments require ongoing maintenance and can induce a high frequency of punctures and potential side effects, gene therapy aims to provide long-term relief from symptoms by enabling the body to produce its own clotting factor.
What challenges does gene therapy for hemophilia face in the current market?
The challenges for gene therapy in the hemophilia market include high treatment costs, patient acceptance, and the need for healthcare providers to become familiar with these new therapies. With prices such as Hemgenix listed at around $3.5 million, reimbursement and market viability can be a concern, potentially limiting access for patients who could greatly benefit from such treatments.
Who can receive gene therapy for hemophilia like Hemgenix?
Gene therapy for hemophilia, such as Hemgenix, is typically indicated for patients with hemophilia B who are experiencing significant bleeding problems despite receiving conventional treatments. Patients are advised to consult with their healthcare providers to determine their eligibility based on their specific health conditions and treatment history.
What is the expected outcome for patients after receiving gene therapy for hemophilia?
Patients receiving gene therapy for hemophilia can expect significant improvements in their ability to produce clotting factor IX. Clinical trials have shown that many patients maintain adequate factor levels long after treatment, reducing or eliminating the need for regular factor infusions. However, individual results may vary, and ongoing monitoring is essential.
How do gene therapy treatments like Hemgenix address the cause of hemophilia?
Gene therapy treatments like Hemgenix directly address the root cause of hemophilia by correcting the genetic mutation responsible for the deficiency in clotting factor. By inserting a normal copy of the clotting factor IX gene into the patients’ liver cells, the therapy enables these cells to produce the clotting factor effectively, thereby preventing bleeding episodes.
What should patients expect during the administration of gene therapy for hemophilia?
During the administration of gene therapy for hemophilia, such as Hemgenix, patients can expect an outpatient procedure that typically lasts a few hours. After undergoing treatment, they will be monitored for potential side effects before being discharged. Overall, the treatment is designed to be safe and minimally invasive, with most patients returning to their regular activities soon after.
What has been the impact of gene therapy on the lives of hemophilia patients?
The impact of gene therapy on the lives of hemophilia patients has been transformative, with many experiencing reduced anxiety about bleeding episodes and a freedom from daily needle regimens. Patients often report improved physical health, greater independence, and an enhanced quality of life, showcasing the potential of gene therapy to revolutionize hemophilia treatment.
| Key Points | Details |
|---|---|
| Gene Therapy Introduction | Terence Blue became the first patient in New England to receive Hemgenix, a new gene therapy for hemophilia B, at Brigham and Women’s Hospital. |
| Living with Hemophilia | Blue has managed hemophilia since childhood, requiring regular treatments with clotting factors to prevent bleeding. |
| Why Gene Therapy Matters | Hemgenix offers a potential long-lasting solution, reducing the need for frequent injections and improving quality of life. |
| Market Challenges | High costs and insurance challenges hinder wider adoption of gene therapies in general due to their one-time treatment nature. |
| Therapy Mechanism | Bioengineered viruses deliver functional copies of the defective gene to liver cells, enhancing production of clotting factor IX. |
| Outcomes of Gene Therapy | After Hemgenix administration, Blue’s factor IX levels increased significantly, indicating effective therapy. |
Summary
Gene Therapy for Hemophilia is a groundbreaking approach that has the potential to transform the lives of patients suffering from this genetic disorder. By utilizing gene therapy, particularly Hemgenix, patients like Terence Blue are experiencing relief from the constant management that comes with hemophilia. This innovative treatment not only enhances clotting factor production but also promises a significant improvement in the quality of life for those affected. As the healthcare landscape evolves, it is crucial to address the challenges of market costs and patient acceptance to fully realize the potential of gene therapy in treating hemophilia.